#include <openbabel/stereo/cistrans.h>
Public Member Functions | |
| Config () | |
| Config (unsigned long _begin, unsigned long _end, const OBStereo::Refs &_refs, OBStereo::Shape _shape=OBStereo::ShapeU) | |
| bool | operator== (const Config &other) const |
| bool | operator!= (const Config &other) const |
Public Attributes | |
Data members defining stereochemistry. | |
| unsigned long | begin |
| unsigned long | end |
| OBStereo::Refs | refs |
| OBStereo::Shape | shape |
| bool | specified |
Detailed Description
Stereochemical configuration for double-bond cis/trans stereochemistry.
The config struct represents the stereochemistry in a well defined way. For cis/trans stereo bonds, the following data members define the spacial arrengement of the atoms.
- OBStereo::Ref
begin:The begin atom for the double bond. - OBStereo::Ref
end:The end atom for the double bond. - OBStereo::Refs
refs:The 4 atoms connected to the double bond. - OBStereo::Shape
shape:The shape formed by therefsby connecting them in the same order as they occur inrefs.
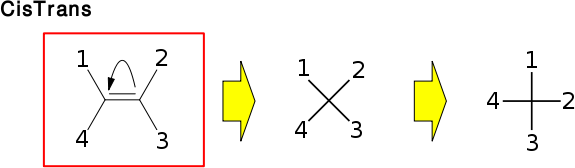
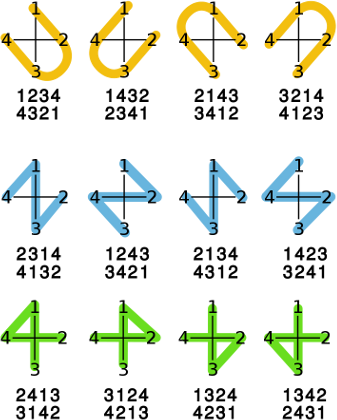
Only begin and end are specific for OBCisTransStereo::Config. The other data members occur in all OBTetraPlanarStereo derived classes.
Constructor & Destructor Documentation
◆ Config() [1/2]
|
inline |
Default constructor. Initializes begin and end to OBStereo::NoRef and shape to OBStereo::ShapeU.
◆ Config() [2/2]
|
inline |
Constructor with all parameters.
- Parameters
-
_begin The double bond begin atom id. _end The double bond end atom id. _refs The 4 reference ids. _shape The shape for the 4 reference ids.
Member Function Documentation
◆ operator==()
| bool operator== | ( | const Config & | other | ) | const |
Equal to operator. Comparing OBCisTransStereo::Config structs is done using the information stored in the struct's data members (i.e. begin, end, refs and shape).
There are a number of cases resuling in false being returned:
beginandenddon't match (is checked using the 2 combinations)- One of the Refs lists does not contain 4 elements.
- 2 or more OBStereo::ImplicitRef values in a single Config struct
- (The two
refsdon't share a single common element)
In the simplest case where both refs contain exactly the same elements (OBStereo::ContainsSameRefs()), coould include OBStereo::ImplicitRef), both Config struct are normalized to OBStereo::ShapeU starting with the same element. After this normalization, there are two possible orientations to overlay the shape on the double bond. From the illustration below, it can be seen only refs[2] has to be checked in order to conclude both Config structs have the same stereochemistry.
1 4 1 4 1------4
\ / | | |
C==C | | |
/ \ | | |
2 3 2------3 2------3
1 2 3 4 1 2 3 4
| | | | <- in any case, refs[0] & refs[2] remain unchanged
1 2 3 4 1 4 3 2
When comparing a Config struct with explicit hydrogen(s) to one with implicit hydrogen(s), both refs are also normalized to OBStereo::ShapeU starting with the same common element. This shared element cannot be OBStereo::ImplicitRef. Depending on the position of the OBStereo::ImplicitRef element(s) in the refs, 3 cases are possible:
refs[2] != OBStereo::ImplicitId: (analog to the case above where they contained the same elements ) 1 2 3 4 | | <- refs[0] & refs[2] remain unchanged 1 H 3 H else: 1 2 3 4 | | <- refs[0] & refs[3] remain unchanged 1 H H 4 1 2 3 4 | | <- refs[0] & refs[1] remain unchanged 1 2 H H
In each case, the orientation of the U shape is also defined since there can be only one OBStereo::ImplicitRef for each side of the double bond.
- Returns
- True if both Config structs represent the stereochemistry.
◆ operator!=()
|
inline |
Not equal to operator. This is the inverse of the Equal to operator==.
- Returns
- True if the two Config structs represent a different stereochemistry.
Member Data Documentation
◆ begin
| unsigned long begin |
◆ end
| unsigned long end |
◆ refs
| OBStereo::Refs refs |
◆ shape
| OBStereo::Shape shape |
The shape of the 4 reference ids.
Referenced by OBCisTransConfig::Convert().
◆ specified
| bool specified |
True if the stereochemistry is specified. When false, the described special orientation is only accidental (i.e. unspecified).
Referenced by OpenBabel::CanonicalLabels(), OBCisTransConfig::Convert(), OBMol::CopySubstructure(), OBBuilder::CorrectStereoBonds(), OBDepict::DrawMolecule(), OpenBabel::findMetalloceneBonds(), and OpenBabel::intToStr().
The documentation for this struct was generated from the following file:
 1.8.13
1.8.13